Get to know the MedRing
The MedRing Platform
Device
MedRing
A self-insertable and removable medical device to be placed into the vagina up to 28 days, to release precise doses of medication according to a personalized delivery schedule. The device can monitor bio signals and collect data to improve the treatment.
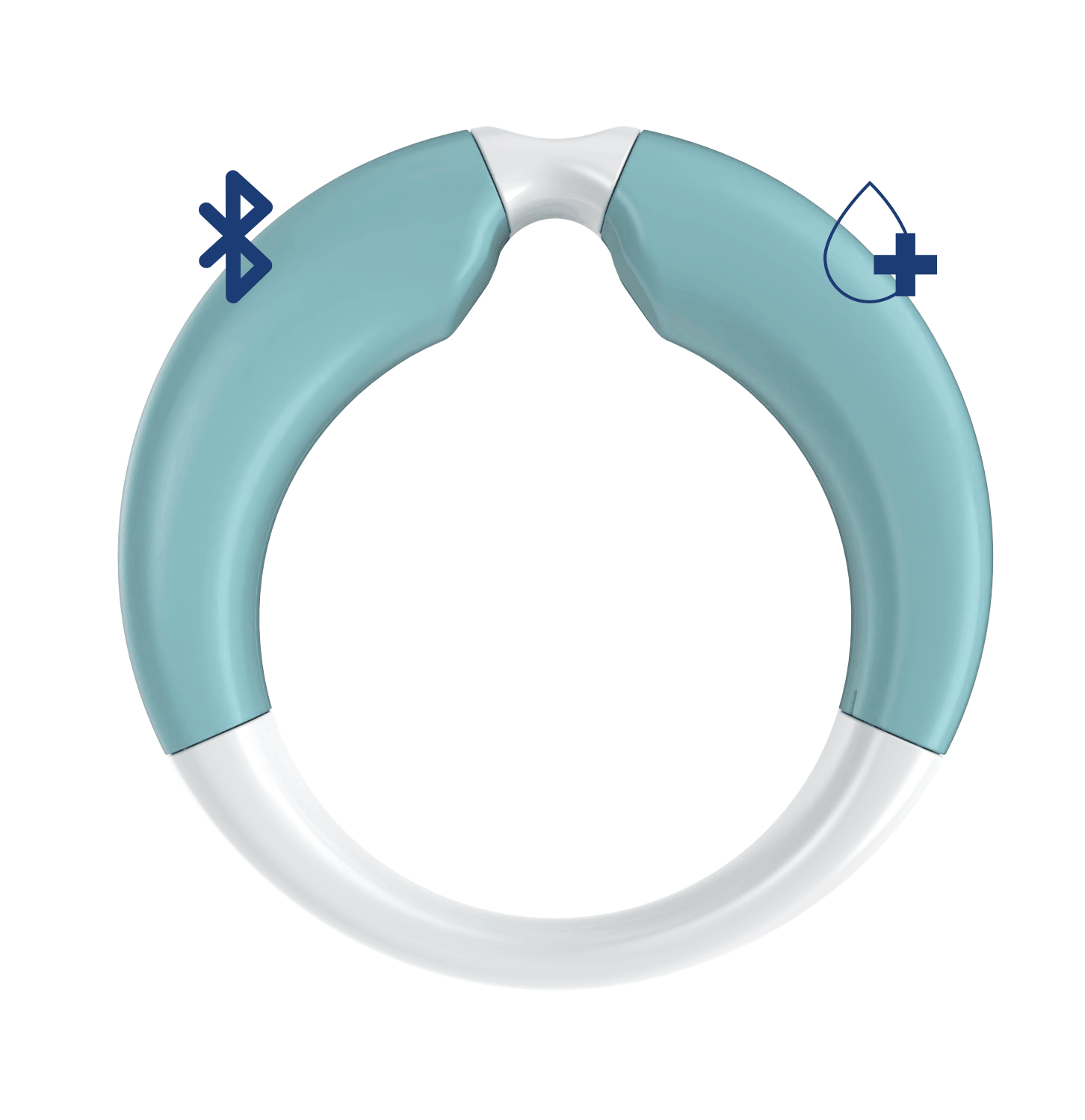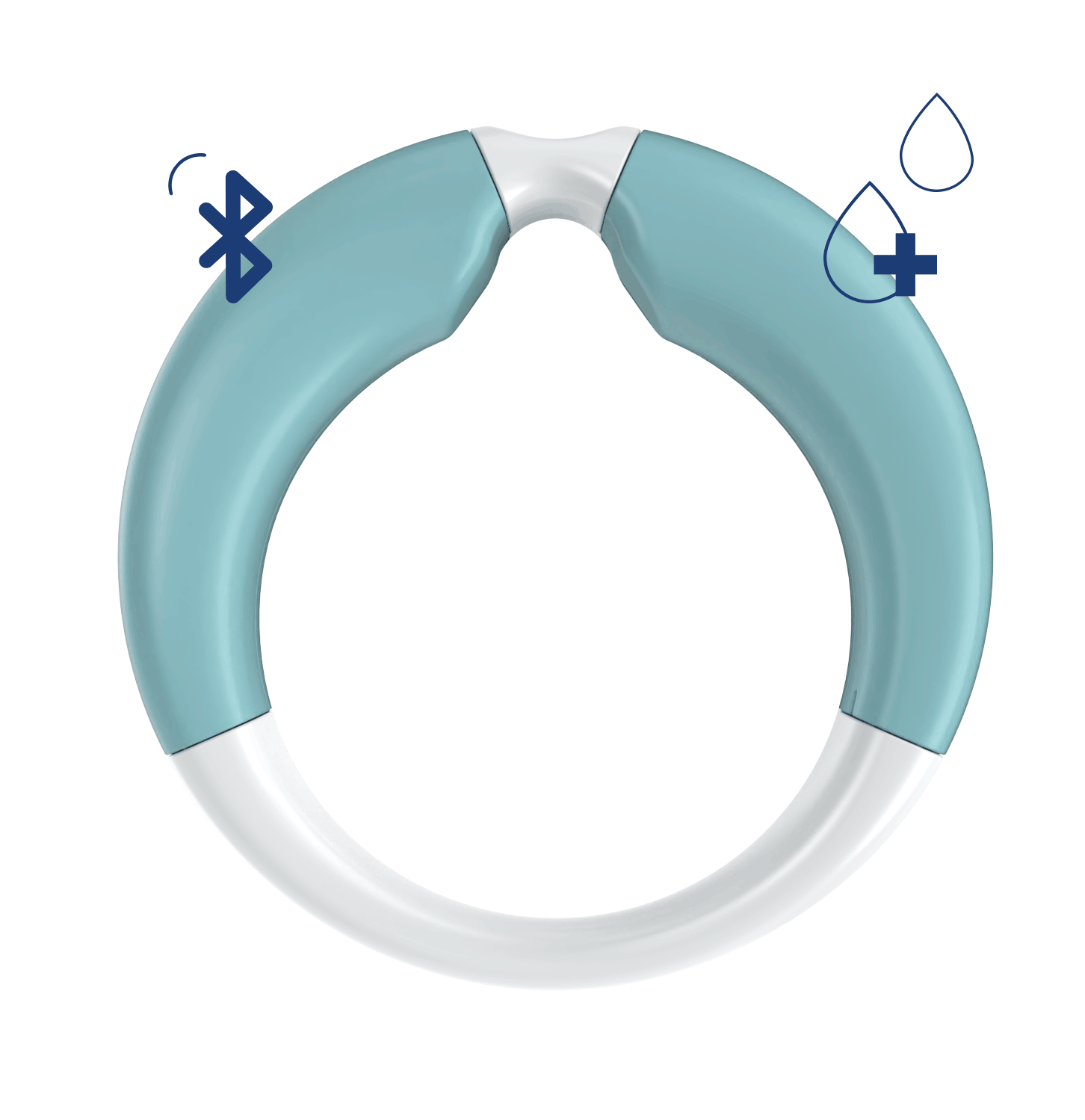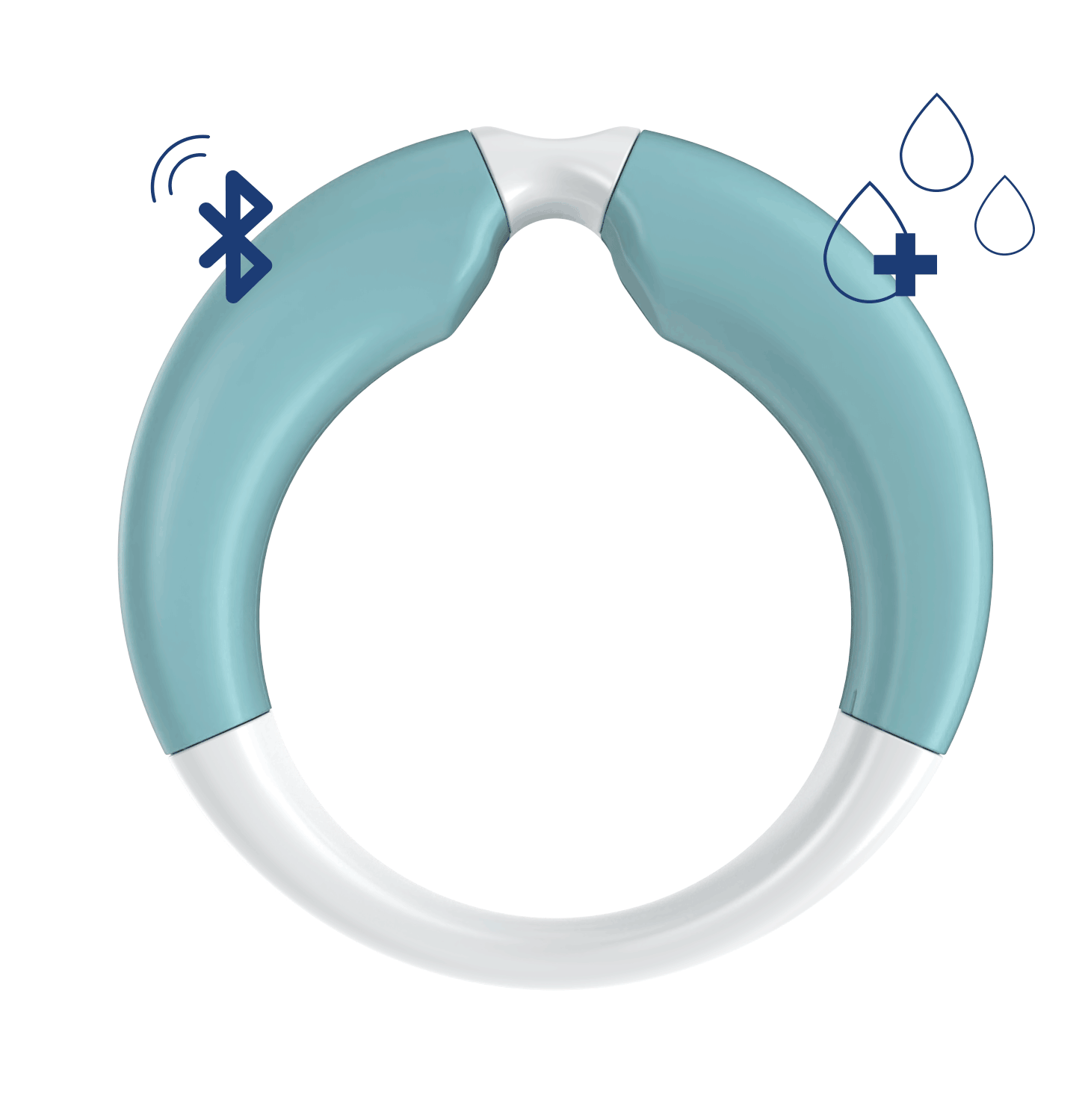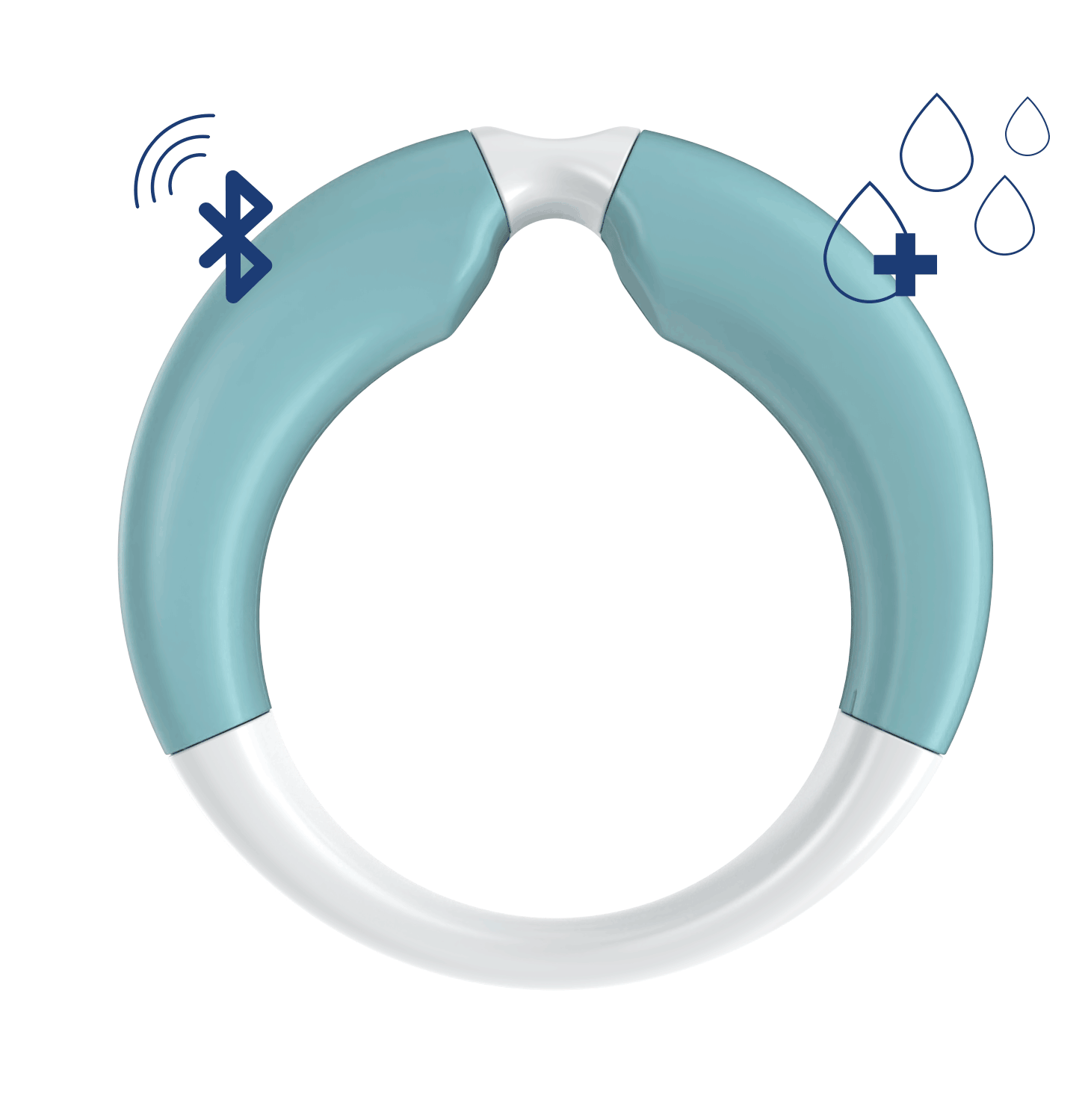
- Shape and dimensions optimized for the vagina
- Flexible materials for self-insertion and removal
- Drug delivery
- Pump for accurate drug administration
- Suitable for a range of Active Pharmaceutical Ingredients (API)
- Vaginal route: high bio-availability and no hepatic first-pass-effect
- Operational lifetime of 28 days
- Temperature measurement (for compliance)
- Bluetooth connection
Smartphone
Companion App
The MedRing device is connected to the smartphone of the user via Bluetooth.
Enabling control through the Companion App without removing the device.
The Companion App provides the status of the device, gives insight into the treatment progress
and allows the personalization of the drug delivery schedule.
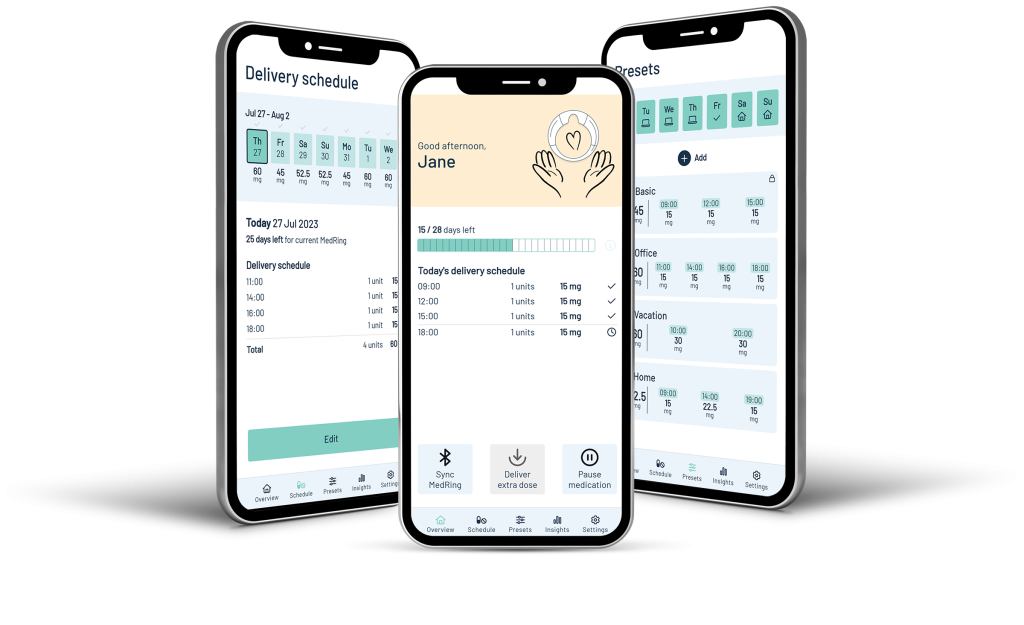
- MedRing device activation
- Communication with the MedRing via Bluetooth
- Schedule time and dose of drug administration
- Collect device data and track treatment progress
- In-app support and use instructions
- Account authentication and secure login
- Compatible with iOS & Android
Cloud
Data Platform
The cloud environment receives data from the Companion App to back-up personal settings, device configuration and treatment data.
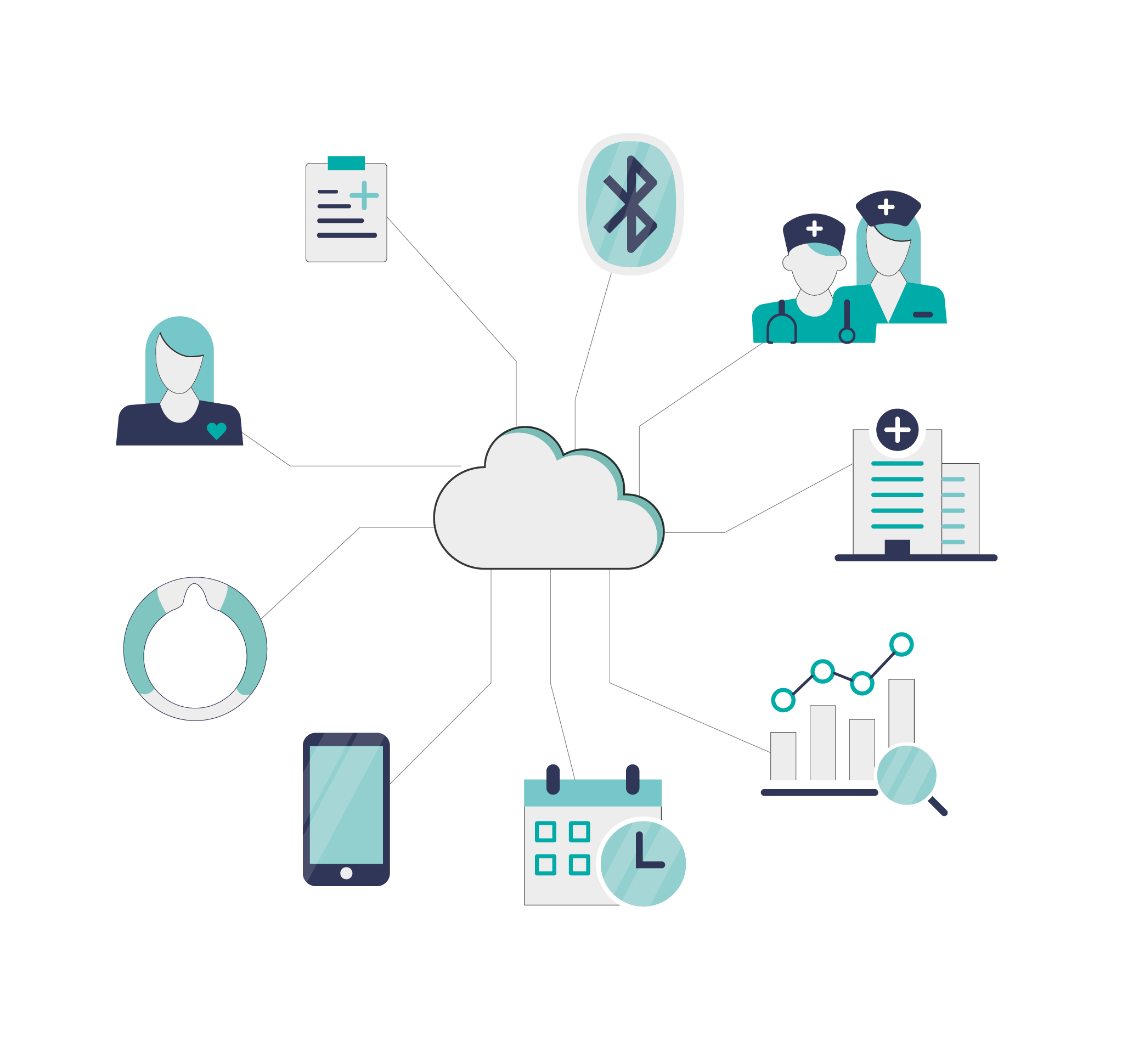
- Account administration
- Authentication of users and devices
- Secure transfer and storage of data
- Data analysis for research and development
Future
Advanced Scheduling
The flexibility in drug administration scheduling offered by the MedRing provides numerous possibilities for advanced treatment.
- Precise titration schedules
- Tapering and chronotherapy
- Drug delivery based on bio signals (closed-loop system)
Future
Diagnostic Opportunities
The continuous monitoring opportunity of the MedRing in the vaginal environment offers unique possibilities and allows for potential diagnostics.
- Discrete 24/7 monitoring
- Long-term remote monitoring
- Treatment effectiveness feedback
- Disease diagnostics
- Platform for wide variety of sensors
Future
Digital Healthcare
The digitalization of drug administration and advanced monitoring provide, with consent of the patient, unprecedented data for the pharmaceutical and medical device industry.
- Medication intake compliance
- Aggregation of healthcare data
- Exchange of data between healthcare systems
- Advanced insights for pharmaceutical and clinical research
Get in touch
Office Address
Leiden Bio Science Park – BioPartner 3
Galileiweg 8, 2333 BD Leiden
The Netherlands
Postal Address
LiGalli B.V.
J.H. Oortweg 21, 2333 CH Leiden
The Netherlands
Disclaimer policy
This website of LiGalli B.V., Leiden, The Netherlands, includes forward looking statements.
These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks, evolving uncertainties and change.
There can be no guarantees, with respect to pipeline products, that they will receive necessary regulatory approval nor that they will prove to be commercially successful.
If underlying assumptions might prove to be inaccurate, or risks or uncertainties materialize, actual situations and results may differ materially from those set forth in the forward looking statements in the LiGalli website.
