Science & Innovation
The MedRing
as a platform
is built upon market needs, medical science, high tech innovations and clinical studies.
The MedRing as a platform has been developed, tested and upgraded ever since 2016. Starting point was general knowledge about vaginal absorption of drugs, the positive results of the vaginal rings for contraconception and need for new reliable, personalized drug administration, made possible by emerging technology. Eventually, the platform offers great opportunities for innovative treatments, monitoring and insight for women’s health.
Following the first prototypes several tests and studies were executed by LiGalli in cooperation with renowned institutes like, CHDR, University of Leiden, Maxima Medical Center. This overview is carefully selected by LiGalli to give a clear view about the possibilities of the MedRing. It contains a small selection of our literature of important studies, articles and insights. If you are intrigued and would like to learn more, more please contact us.
Publications LiGalli
January 2026
Peltenburg, S. I. et al. (2026) ‘Introducing personalized patient care in overactive bladder management using the MedRing OAB system for intravaginal oxybutynin administration’, Drug Delivery, 33(1). doi: 10.1080/10717544.2026.2617683.
This usability study demonstrated that the MedRing for Overactive Bladder is a feasible and safe alternative for oral oxybutynin administration over a period of 28 days.
Moreover, intravaginal dosing - including flexible, nightly and on-demand dosing - resulted in rapid absorption and sufficient oxybutynin levels.
January 2026
Veen, J. et al. (2026) ‘User acceptability and ease of use of the MedRing, a new personalized vaginal drug delivery and monitoring device’, Expert Opinion on Drug Delivery, pp. 1–8. doi: 10.1080/17425247.2026.2619095.
This acceptability study demonstrated that the MedRing was well-tolerated, highly accepted, comfortable to wear, and easy to insert and remove.
February 2023
de Laat, W. et al. (2023) ‘First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring’, Drug Delivery, 30(1). doi: 10.1080/10717544.2023.2180113.
This clinical study demonstrated that intravaginal administration of oxybutynin hydrochloride using the MedRing device has a good safety profile and is well tolerated.
Proof of concept
The MedRing: A revolutionary Intravaginal Drug Administration System
Vaginal administration
- Low dosage
- Less side effects
- High compliance
- Long term administration possibilities
Complex and precise administration
- 28 days
- Up to 6 deliveries per day
- Flexible scheduling
- Noctural deliveries
Monitoring and data collection
- Temperature monitoring
- Treatment insights
- Personal preferences
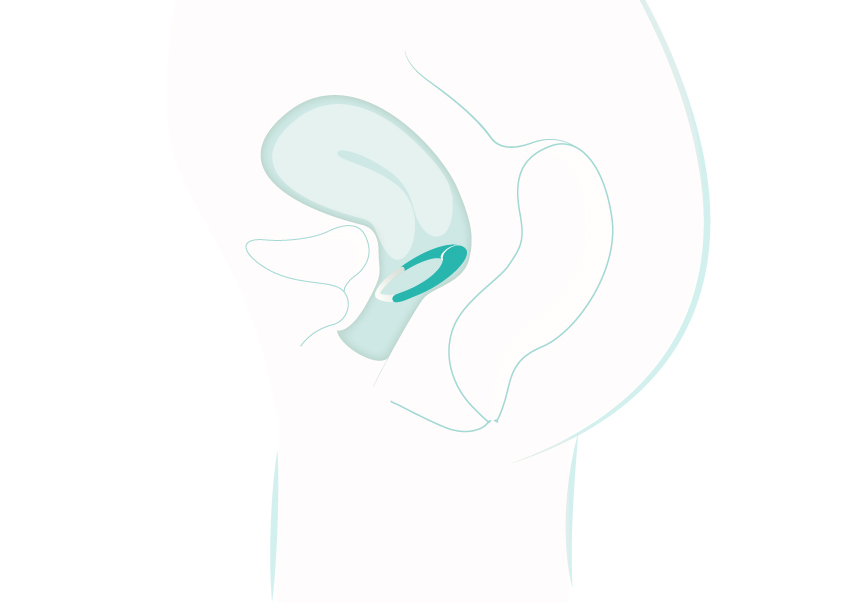
The MedRing introduces an innovative method for intravaginal drug administration. Due to its rich vascularization, the vaginal route provides an effective means for rapid systemic absorption while bypassing the hepatic first-pass metabolism associated with oral administration. By bypassing the first-pass metabolism the MedRing can increase bioavailability of drugs versus oral administration. This can lead to lower doses and less side-effects, as we have seen in our trials with oxybutynin.
The MedRing is anatomically designed to achieve optimal fit within the vaginal cavity, ensuring direct and continuous contact with the vaginal mucosa. This facilitates absorption of the active pharmaceutical ingredient, accompanied by the intuitive smartphone Companion App, the MedRing empowers users to personalize dosing schedules and monitor therapy effectiveness. This makes it the first user-customizable, controlled intravaginal drug delivery platform, ushering in a new era of precision medicine for women’s health.
Other Scientific Evidence
01
Srikrishna, S et al. (2013) ‘The vagina as a route for drug delivery: a review‘, Int Urogynecol J., 24:537–543. doi: 10.1007/s00192-012-2009-3
Vaginal administration allows nondaily, low, continuous dosing, which results in stable drug levels and may, in turn, achieve a lower incidence of side effects and improve patient compliance”.
02
Wieder, D.R. et al. (2010) ‘Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing®)‘, International Journal of Women’s Health, 2 401–409 . doi: 10.2147/IJWH.S6162
Overall, the contraceptive vaginal ring appears to be very effective, with a favorable side-effect profile, and is highly acceptable to most patients.
03
Bypass of the first pass effect allows for greater bioavailability, lower dosing with accompanying less side effects and less drug-drug interference.
04
Ridgeway, K. et al. (2022) ‘Vaginal ring acceptability: A systematic review and meta-analysis of vaginal ring experiences from around the world‘, Contraception, 106:16-33. doi: 10.1016/j.contraception.2021.10.001
Women who used vaginal rings reported they were acceptable across indications geographic regions and indications.
Get in touch
Office Address
Leiden Bio Science Park – BioPartner 3
Galileiweg 8, 2333 BD Leiden
The Netherlands
Postal Address
LiGalli B.V.
J.H. Oortweg 21, 2333 CH Leiden
The Netherlands
Disclaimer policy
This website of LiGalli B.V., Leiden, The Netherlands, includes forward looking statements.
These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks, evolving uncertainties and change.
There can be no guarantees, with respect to pipeline products, that they will receive necessary regulatory approval nor that they will prove to be commercially successful.
If underlying assumptions might prove to be inaccurate, or risks or uncertainties materialize, actual situations and results may differ materially from those set forth in the forward looking statements in the LiGalli website.